Asymmetric induction in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment . Asymmetric induction is a key element in asymmetric synthesis.
Asymmetric induction was introduced by Emil Fischer based on his work on carbohydrates . Several types of induction exist.
Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries.In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.
Several models exist to describe chiral induction based on a combination of steric and electronic considerations and often in conflict with each other. Models have been devised by Cram (1952), Cornforth (1959), Felkin (1969) and others.
Cram's rule of asymmetric induction
The Cram's rule of asymmetric induction developed by Donald J. Cram in 1952 is an early concept relating to the prediction of stereochemistry in certain acyclic systems. In full the rule is:
In certain non-catalytic reactions that diastereomer will predominate which could be formed by the approach of the entering group from the least hindered side when the rotational conformation of the C-C bond is such that the double bond is flanked by the two least bulky groups attached to the adjacent asymmetric center.
The rule indicates that the presence of an asymmetric center in a molecule induces the formation of an asymmetric center adjacent to it based on steric hindrance.
In his 1952 publication Cram presented a large number of reactions described in the literature for which the conformation of the reaction products could be explained based on this rule and he also described an elaborate experiment (scheme 1) making his case.
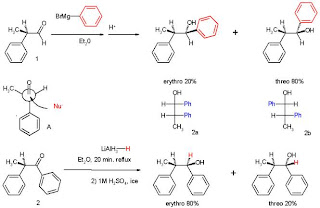
The experiments involved two reactions. In experiment one 2-phenylpropionaldehyde (1, racemic but (R)-enantiomer shown) was reacted with the Grignard reagent of bromobenzene to 1,2-diphenyl-1-propanol (2) as a mixture of diastereomers, predominantly the threo isomer (see for explanation the Fischer projection).
The preference for the formation of the threo isomer can be explained by the rule stated above by having the active nucleophile in this reaction attacking the carbonyl group from the least hindered side (see Newman projection A) when the carbonyl is positioned in a staggered formation with the methyl group and the hydrogen atom, which are the two smallest substituents creating a minimum of steric hindrance, in a gauche orientation and phenyl as the most bulky group in the anti conformation.
The second reaction is the organic reduction of 1,2-diphenyl-1-propanone 2 with lithium aluminum hydride which results in the same reaction product as above but now with preference for the erythro isomer (2a). Now a hydride anion (H-) is the nucleophile attacking from the least hindered side (imagine hydrogen entering from the paper plane).
In the original 1952 publication additional evidence was obtained for the structural assignment of the reaction products by applying them to a Chugaev elimination where the threo isomer reacts to the cis isomer of -α-methyl-stilbene and the erythro isomer to the trans version.
Felkin model
The Felkin model (1968) named after Hugh Felkin also predicts the stereochemistry of nucleophilic addition reactions to carbonyl groups [4]. Felkin argued that the Cram model suffered a major drawback: an eclipsed conformation in the transition state between the α-carbonyl substituent (the hydrogen atom in aldehydes) and the largest β-carbonyl substituent. He demonstrated that by increasing the steric bulk of the α-substituent from methyl to ethyl to isopropyl to isobutyl, the stereoselectivity also increased which is not predicted by Cram's rule:

The Felkin rules are:
The transition states are reactant-like.
Torsional strain (Pitzer strain) involving partial bonds (in transition states) represents a substantial fraction of the strain between fully-formed bonds, even when the degree of bonding is quite low. The conformation in the TS is staggered and not eclipsed with the substituent R skew with respect to two adjacent groups one of them the smallest in TS A.
Torsional strain (Pitzer strain) involving partial bonds (in transition states) represents a substantial fraction of the strain between fully-formed bonds, even when the degree of bonding is quite low. The conformation in the TS is staggered and not eclipsed with the substituent R skew with respect to two adjacent groups one of them the smallest in TS A.

For comparison TS B is the Cram transition state.
The main steric interactions involve those around R and the nucleophile but not
the carbonyl oxygen atom.
A polar effect or electronic affect stabilizes a transition state with maximum separation between the nucleophile and an electron-withdrawing group. For instance haloketones do not obey Cram's rule and in the example above replacing the electron-withdrawing phenyl group by a cyclohexyl group reduces stereoselectivity considerably.
The Felkin-Anh model is an extension of the Felkin model. A so-called Felkin product is that reaction product that obeys the Felkin-Anh model and an anti-Felkin product obviously does not.
1 comments:
Professor Prem raj Pushpakaran ♡ പ്രൊഫസ്സർ പ്രേം രാജ് പുഷ്പാകരന് ♡ writes -- 2019 marks the centenary birth year of Donald J. Cram, who was one of the founder of the field of host–guest chemistry & Cram's rule of asymmetric induction!!!
Post a Comment