Related tags: chemical analysis, syllabus questions, Mass spectroscopy
PAPER 1– SECTION A
1. General information on science and its interface with society to test the candidate’s awareness of science, aptitude of scientific and quantitative reasonsing.
2. COMMON ELEMENTRY COMPUTER SCIENCE ( Applicable to all candidates offering subject areas ).
3. History of development of computers, Mainframe, Mini, Micro’s and Super Computer Systems.
4. General awareness of computer Hardware i..e. CPU and other peripheral devices ( input / output and auxiliary storage devices ).
5. Basic knowledge of computer systems, software and programming languages i.e. Machine language, Assembly language and higher level language.
6. General awareness of popular commercial software packages like LOTUS, DBASE, WORDSTAR, other Scientific application packages.
PAPER 1 – SECTION B
1. Structure and Bonding : Atomic orbitals, electronic configuration of atoms ( L – S coupling ) and the periodic properties of elements ; ionic radii, ionization potential, electron affinity, electronegativity; concept of hybridization. Molecular orbitals and electronic configuration of homonuclear and heteronucelar diatomic molecules. Shapes of polyatomic molecules; VSEPR, theory. Symmetry elements and point groups for simple molecules. Bond lengths, bond angles, bond order and bond energies. Types of Chemical Bond ( weak and strong ) intermolecular forces, structure of simple ionic and covalent solids, lattice energy.
2. Acids and Bases : Bronsted and Lewis acids and bases, pH and pKa, acid-based concept in non-acqueous media ; HSAB concept. Buffer solution.
3. Redox Reactions : Oxidation numbers, Redox potential, Electrochemical series, Redox indicators.
4. Energetics and Dynamics of Chemical Reactions : Law of conservation of energy, Energy and entheipy of reactions. Entropy, free-energy, relationship between free energy change and equilibrium. Rates of chemical reactions (first-and second-order reactions). Arrhenius equation and concept of transition state. Mechanisms, including SN 1 and SN 2 reactions, electron transfer reactions, catalysis. Coiligative properties of solutions.
5. Aspects of s, p, d, f, Block Elements : General characteristics of each block. Chemical principles involved in extractions and purification of iron, copper, lead, zinc and aluminium. Coordination chemistry; structural aspects, isomerism, octahedral and tetrahedral crystal – field splitting of dorbitals. CFSE, magnetism end colour of transition metal ions. Sandwich compounds, metal carbonyls and metal clusters. Rare gas compounds, non-stoichiometric oxides. Radio activity and transmutation of elements, isotopes and their applications.
6. IUPAC Nomenciature of Simple Organic and Inorganic Compounds.
7. Concept of Chirality : Recognition of symmetry elements and chiral structures; R – S nomenciature, diastereoisomerism in acyclic and cyclic systers; E – Z isomerisms. Conformational analysis of simple cyclic ( chair and boat cyclo hexanes ) and acyclic systems. Interconversion of Fischer, Newman and Sawhorse projections.
8. Common Organic Reactions and Mechanisms : Reactive intermediates, Formation and stability of carbonium ions, carbanians, carbenes, nitrenes, radicals and arynes. Nucleophilic, electrophilic, radical substitution, addition and elimination reactions. Familiar name reactions : Aldol, Perkin, Stobbe, Dieckmann condensations; Holmann, Schmidt, Lossen, Curtius, Backmann and Fries rearrangements; Reimer – Tiemann, Reformatsky and Grignard reactions. Diels – Aider reactions; Clasien rearrangements; Friedeal – Crafts reaftions; Witting reactions; and robinson annulation. Routine functional group transformations and interconversions of simple functionalities. Hydroboration, Oppenaur oxidations; Clemmensen, Wolff-Kishner, Meerwein – Ponndorf – Verley and Birch reductions.
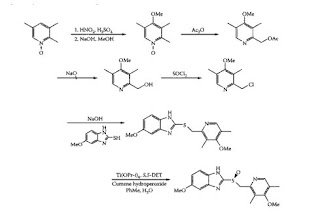
9. Elementary principles and applications of electronic, vibrational, NMR, EPR and Mass Spectral techniques to simple structural problems.
10. Data Analysis : Types of errors, propagation of errors, accuracy and precision, least-squares analysis, average standard deviation.
11. Statistical Tharomodynamics : Thermodynamic probability and entropy; Maxwell – Boltzmann, Bose – Einstein and Fermi – Dirac statistics. Partition function; rotational translational, vibratioanl and electronic partition functions for diatomic molecules; calculations of thermodynamic functions and equilibrium constants. Theories of specific heat for solids.
12. Non-equilibrium Thermodynamics : Postulates and methodologies, linear laws, Gibbs equation, Onsager reciprocal theory.
13. Reaction Kinetics : Methods of determining rate laws. Mechanisms of photochemical, chain and oscillatory reactions. Collision theory of reaction rates; steric factor, treatment of unimolecular reactions. Theory of absolute reaction rates, comparison of results with Eyring and Arrhenius equations, ionic reactions; salt effect. Homogeneous catalysis and Michaelis – Menten kinetics; heterogeneous catalysis.
14. Fast Reaction : Luminescence and Energy transfer processes. Study of kinetics by stopped flow technique, relazation method, flash photolysis and magnetic resonance method.
15. Macromolecules : Number – average and weight average molecular weights ; determination of molecular weights. Kinetics of polymerization. Stereochemistry and mechanism of polymerization.
16. Solids : Dislocation in solids, Schottky and Frenkel defects, Electrical properties; insulators and semiconductors; superconductors, band theory of solids, Solid-state reactions.
17. Nuclear Chemistry : Radioactive decay and equilibrium. Nuclear reactions ; Q value, cross sections, types of reactions, Chemical effects of nuclear transformations; fission and fusion, fission products and fission yields. Radioactive technique; tracer technique, neutron activation analysis, counting techniques such as G. M. ionization and proportional counter.
18. Chemistry of Non-transition Elements : General discussion on the properties of the non-transition elements; special features of individual elements; synthesis, properties and structure of their halides and oxides, polymorphyism of carbon, phosphorus and sulphur. Synthesis, properties and structure of boranes, carboranes, borazines, silicates carbides, silicones, phosphazenes, sulphur-nitrogen compounds; peroxo compounds of boron, carbon and sulphur; oxy acids of nitrogen, phosphours, sulphur and halogens, interhalogens pseudohalides and noble gas compounds.
19. Chemistry of Transition Elements : Coordination chemistry of transition metal ions ; Stability constants of complexes and their determination; stabilization of unusual oxidation states. Stereochemistry of coordination compounds. Ligandfield theory, splitting of d-orbitals in low-symmetry environments. Jahn – Teller effect; interpretation of electronic spectra including charge transfer spectra ; spectrochemical series, nephelauxetic series ,Magnetism; Dia-, para-, ferro- and antiferromagnetism, quenching of orbital angular moment, spinorbit copling, inorganic reaction mechanisms; substitution reactions, trans effect and electron transfer reactions, photochemical reaction of chromium and ruthenium complexes. Fluxional molecules iso-and heteropolyacids ; metal clusters. Spin crossover in coordination compounds.
20.Chemistry of Lanthanides and Actinides : Spectral and magnetic properties; Use of lanthanide compounds as shift reagents.
21. Organometallic Chemistry of Transition Elements : Synthesis, structure and bonding, organometallic reagents in organic synthesis and in homogeneous catalytic reactions ( hydrogenation, hydroformayalation, isomerisation and polymerization ); pl-acid metal complexes, activation of small molecules by coordination.
22. Topics in Analytical Chemistry : Adsorption partition, exclusion electrochromatography, Solvent extraction and ion exchange, methods. Application of atomic and molecular absorption and emission spectroscopy in quantitative analysis Light scattering techniques including nephelometry and Raman spectroscopy. Electroalytical techniques: voltammetry, cyclit, voltammetry, polarography, amperometry, coulometry and comductometry ion-elective electrodes. Annodic stripping voltammetry; TGA, DTA, DSC and online analysors.
23. Bioinorganic Chemistry : Metal ions in Biology, Molecular mechanism of ion transport across membranes; ionophores. Photosynthesis, PSL, PSH; nitrogen fixation, oxygen uptake proteins, cytochromes and ferrodoxins.
24. Aromaticity : Huckel’s rule and concept of aromaticty (n) annulences and heteroannulenes, fulterenes (C60).
25. Stereochemistry and conformational Analysis : Nwere method of asymmetric synthesis ( including enzymatic and catalytic nexus ), enantio and diastereo selective synthesis. Effects of conformation on reactivity in acyclic compounds and cyclohexanes.
26. Selective Organic Name Reactions : Favorskli reaction; Stork enamine reaction; Michael addition, Mannich Reaction; Sharpless asymmetric epoxidation; Ene reaction, Barton reaction, Holmann-Loffer-Freytag reaction, Shapiro reaction, Baeyer-Villiger reaction, Chichibabin reaction.
27. Mechanisms of Organic Reactions : Labelling and Kinetic isotope effects, Hamett equation, ( sigma-rho ) relationship, non-classical carbonium ions, neighbouring group participation.
28. Pericyclic Reactions : Selection rules and stereochemistry of electrocyclic reactions, cycloaddition and sigmatropic shifts, Sommelet, Hauser, Cope and Claisen rearrangements.
29. Heterocyclic Chemistry : Synthesis and reactivity of furan, thiophene, pyrrole, pyridine, quinoline, isoquinoline and indole; Skraup synthesis, Fisher indole synthesis.30. Reagents in Organic Synthesis : Use of the following reagents in organic synthesis and functional group transformations ; Complex metal hybrids, Gilman’s reagent, lithium dimethycuprate, lithium disopropylamide (LDA) dicyclohexylcarbodimide. 1,3 – Dithiane (reactivity umpolung), trimethysilyl iodide, tri-n-butyltin hybride, Woodward and provost hydroxylation, osmium tetroxide, DDQ, selenium dioxide, phase transfer catalysts, crown ethers and Merrified resin, Peterson’s synthesis, Wilkinson’s catalyst, Baker yeast.
31. Chemistry of Natural Products : Familiarity with methods of structure elucidation and biosynthesis of alkaloids, terponoids, steroids, carbohydrates and proteins.
32. Bio-organic Chemistry : Elementry structure and function of biopolymers such as proteins and nucleric acids.
33. Photochemistry : Cis – trans isomeriation, Paterno – Buchi reaction, Norrish Type I and II reactions, photoreduction of ketones, di-pimethane rearrangement, photochemistry of areanes.
34. Spectroscopy : Applications of mass, UV – VIS, IR and NMR spectroscopy for structural elucidation of compound.